Case of the Week #548
1) Radiologist, Imagerie Capricorne, Reunion Island; 2) Obstetrician, Felix Guyon Hospital, Reunion Island; 3) Pathologist, Felix Guyon Hospital, Reunion Island; 4) Fetopathologist, Robert Debre Hospital, Paris, France; 5) Geneticist, Felix Guyon Hospital, Reunion Island; 6) Molecular Geneticist, Necker Hospital, Paris, France; 7) Radiologist, Women's & Children Hospital, Lyon, France
Posting Dates: November 1 - November 14, 2021
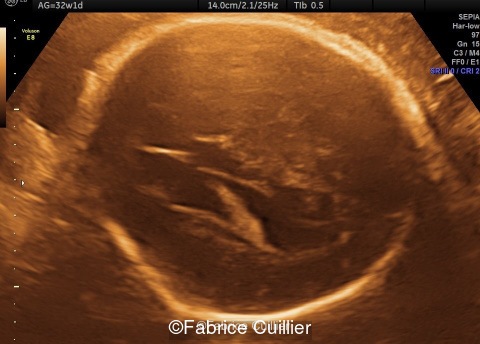
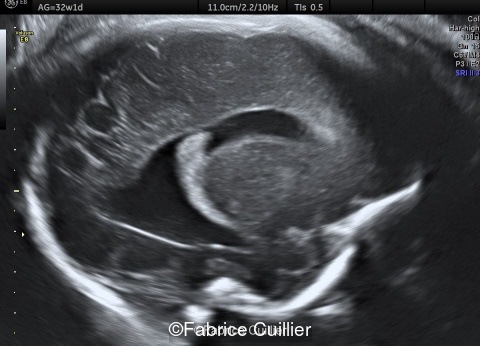
18-year-old primigravida, presented without significant medical or family history. The pregnancy was not a result of consanguineous marriage and prenatal screening for Down syndrome was normal. First and second trimester ultrasound examinations were both normal. Third trimester ultrasound at 32 weeks gestation showed an intracranial unilateral left ventricular dilation of 14mm. Fetal growth, cord doppler and amniotic fluid were normal. There was no other obvious extracranial or intracranial anomalies on ultrasound.
We therefore performed a fetal brain MRI at 32 weeks gestation.
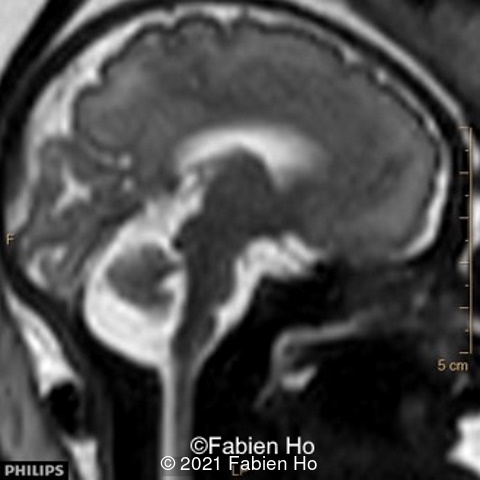
We performed again a fetal brain MRI at 35WG.
View the Answer Hide the Answer
Answer
Our examinations showed the following findings:
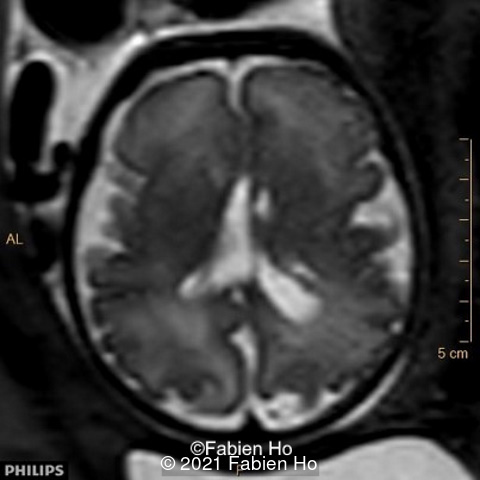
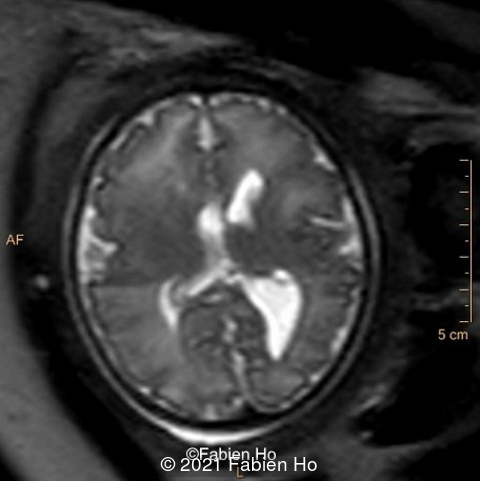
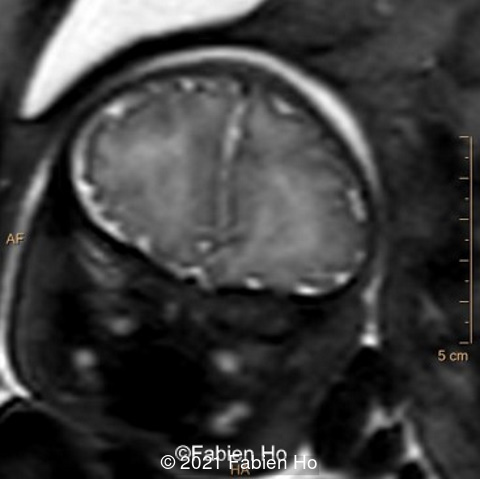
-Images 1,2,3 : ultrasound only showed unilateral left ventricular dilation of 14mm without other obvious brain anomalies.
Fetal MRI incidentally found additional anomalies at 32 weeks gestation including:
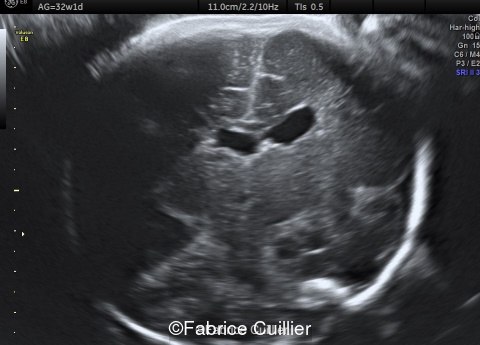
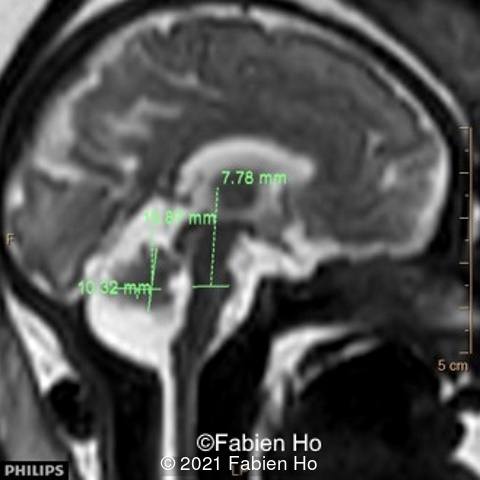
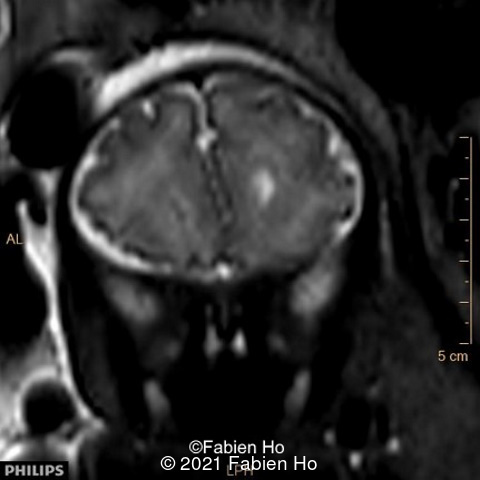
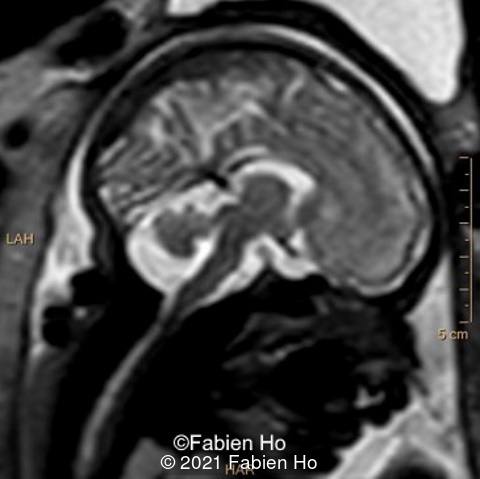
-Images 4,5,6: Hypoplasia of the Pons. Pons biometry was <3rd percentile according to C. Garel biometry. Vermian height and anteroposterior diameters were at the 3rd percentile.
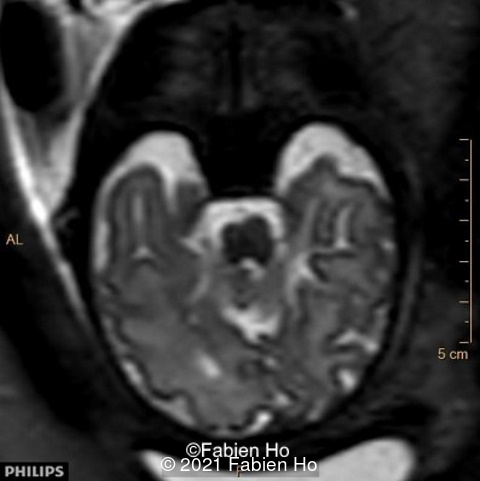
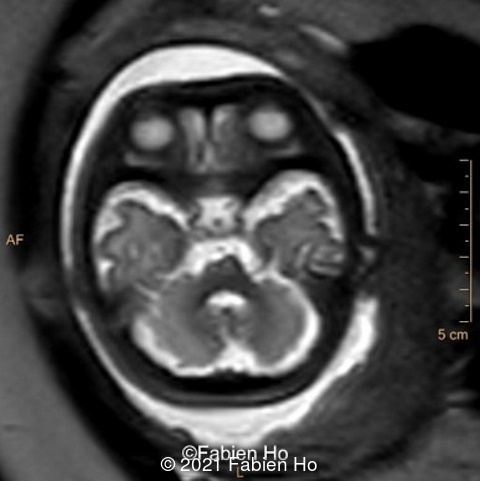
-Image 7: asymmetric and unusual appearance of the pons in axial view.
-Image 8 and 9: distorted anterior interhemispheric fissure. Supratentorial brain biometry was normal. Gyration looked appropriate for 32 weeks gestation.
-Other cerebral structures such as the corpus callosum, optic chiasm, olfactive bulbs were normal (not shown)
-Cerebellum biometry and morphology were normal (not shown).
-Pericerebral spaces were normal and not enlarged.
-No evidence for a clastic event, including on T1w and T2*w sequences (not shown)
We therefore suspected a fetal neurological disease with hypoplasia of the pons. We hypothesized that this was non-syndromic given that only brain anomalies were detected, and no other extra-neurologic anomalies were found on ultrasound. Although these fetal MRI features were non-specific, considered separately, their association (Pons hypoplasia + Pons asymmetric appearance on axial view + distorted interhemispheric fissure) was suggestive of a Tubulinopathy as described by Cabet et al [1].
Our prenatal diagnosis was therefore a Tubulinopathy, and as possible differential diagnoses other diseases involving Pons hypoplasia.
We performed an amniocentesis to rule out chromosomal anomaly (though unlikely as this did not appear to be a syndromic condition), and Cytomegalovirus (CMV) infection. Karyotype was normal, and PCR for CMV in the amniotic fluid was negative. There is no Zika virus on Reunion Island and the patient did not travel overseas. There was neither ultrasound nor doppler evidence for an infectious condition.
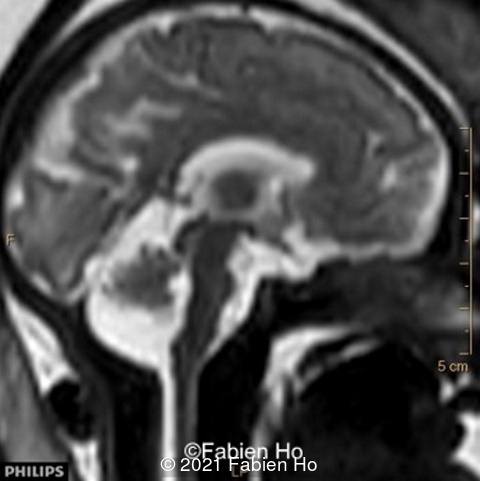
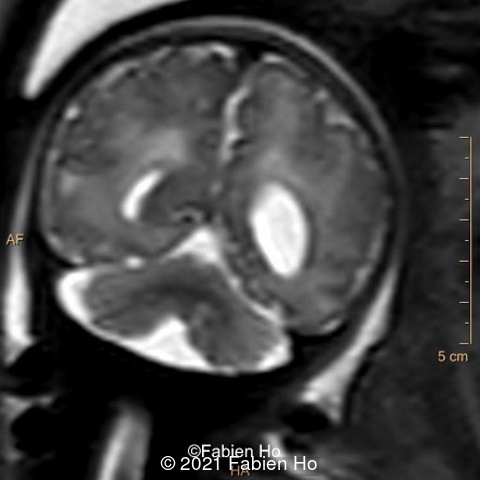
We repeated a fetal MRI at 35 weeks gestation, which showed the same findings, including Pons hypoplasia (image 10), Pons asymmetric appearance on axial view (image 11), distorted interhemispheric fissure (image 13), and unilateral left dilation (image12). Pons and vermis biometry remained at the <3rd percentile, while supratentorial brain biometry was normal. Gyration seemed normal, though there was suspicion for polymicrogyria in the inner temporal left lobe on image 14.
Prenatal counselling predicted a poor prognosis due to the pons hypoplasia and suspicion of tubulinopathy. The parents chose termination of pregnancy, and consented to additional pathologic and genetic investigations.
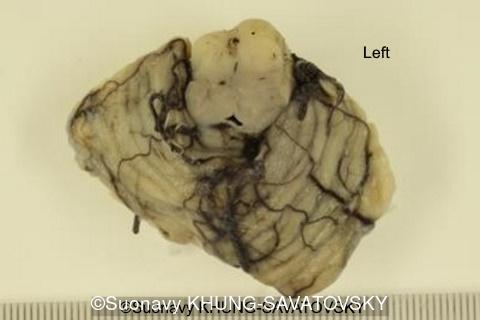
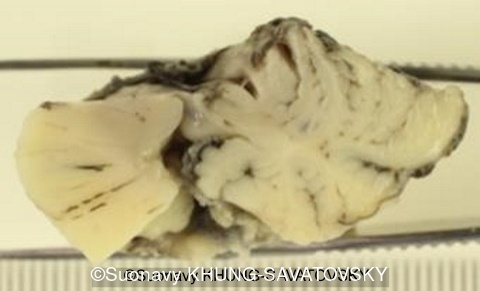
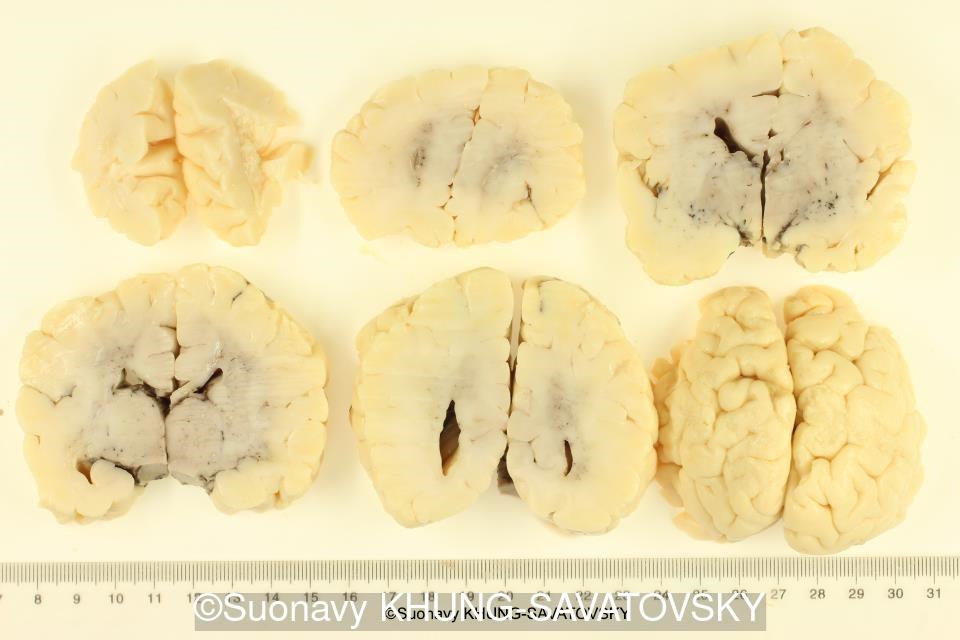
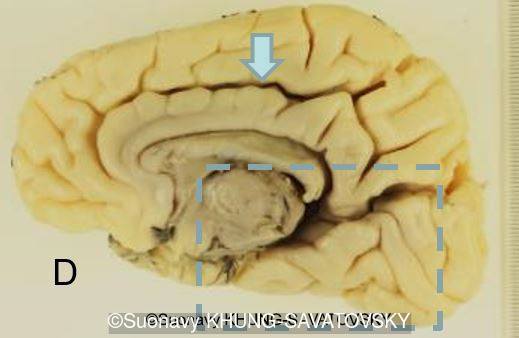
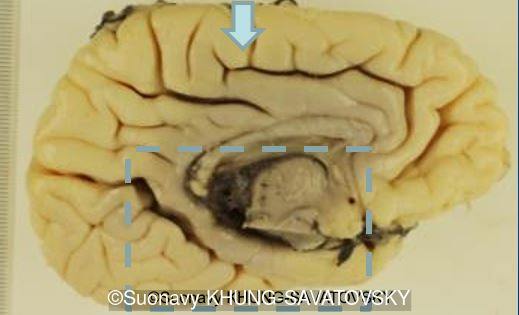
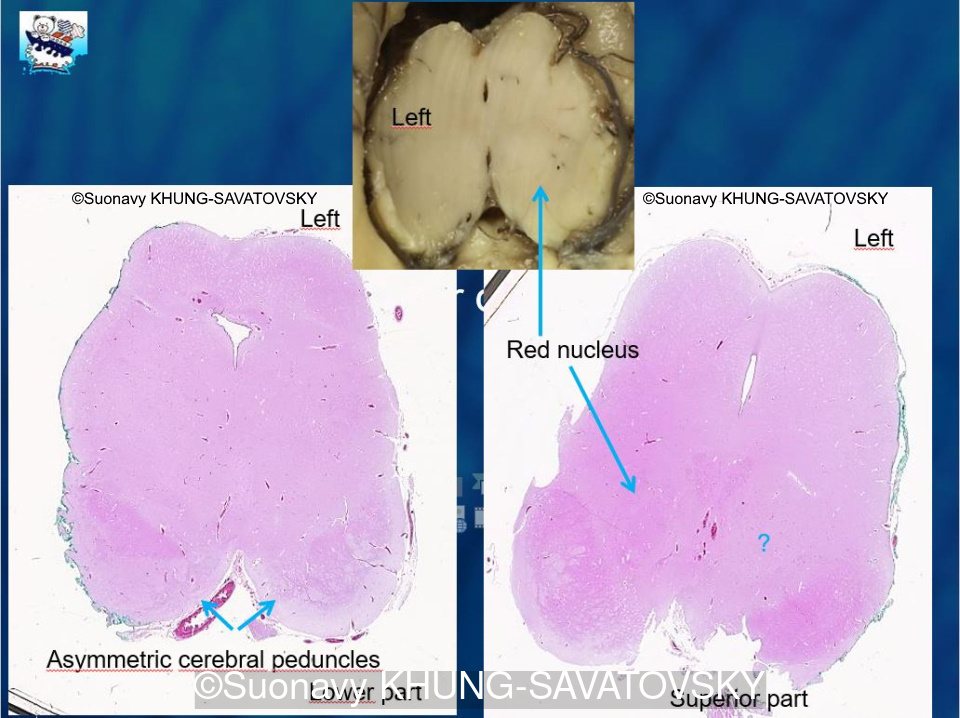
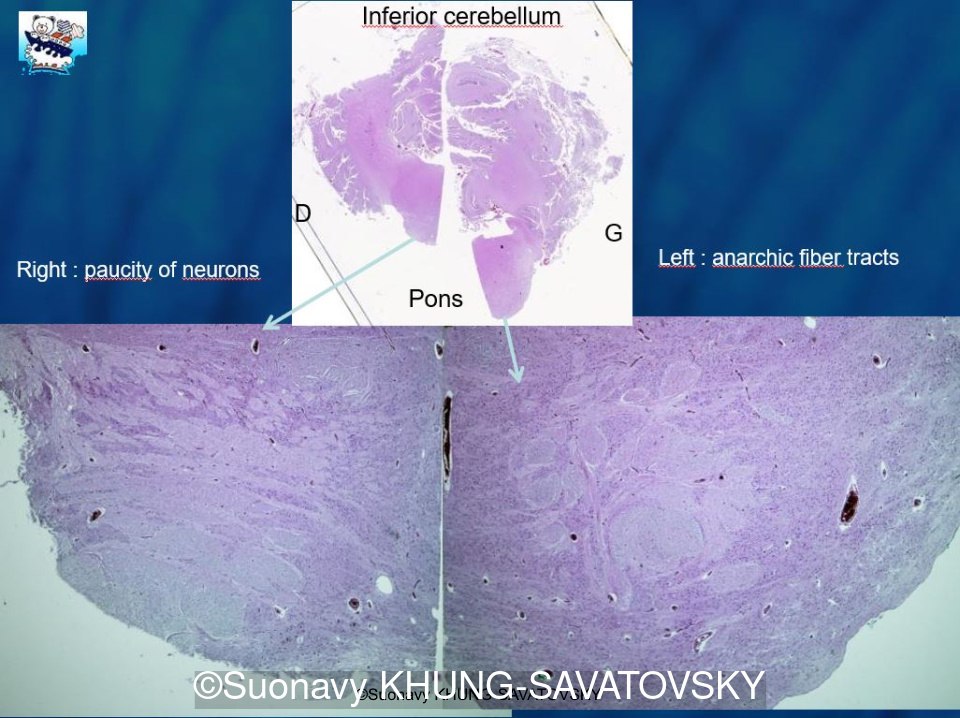
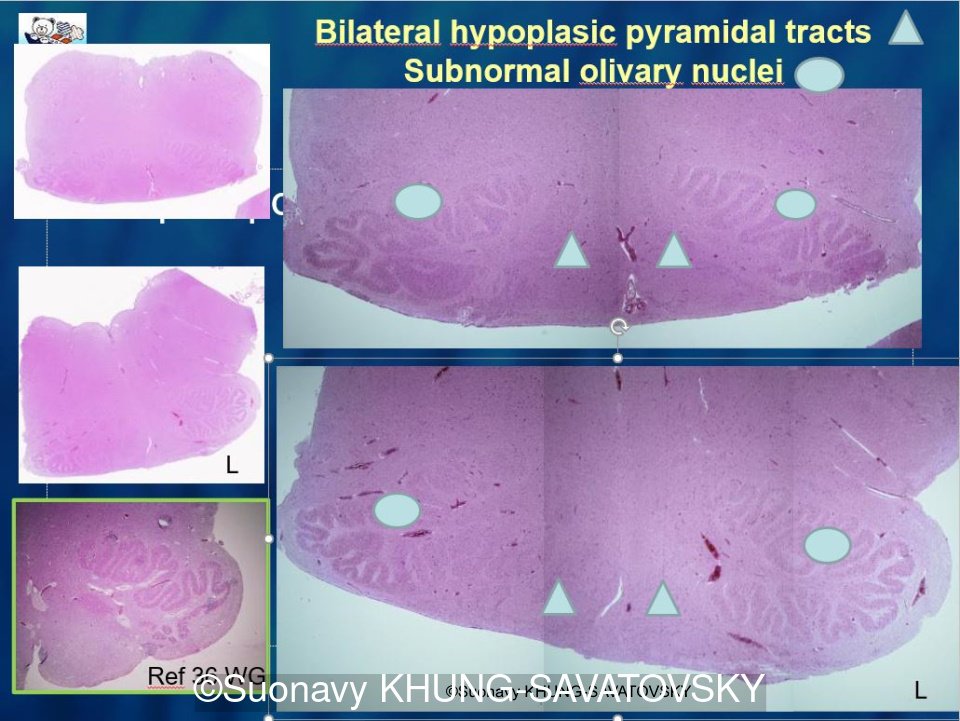
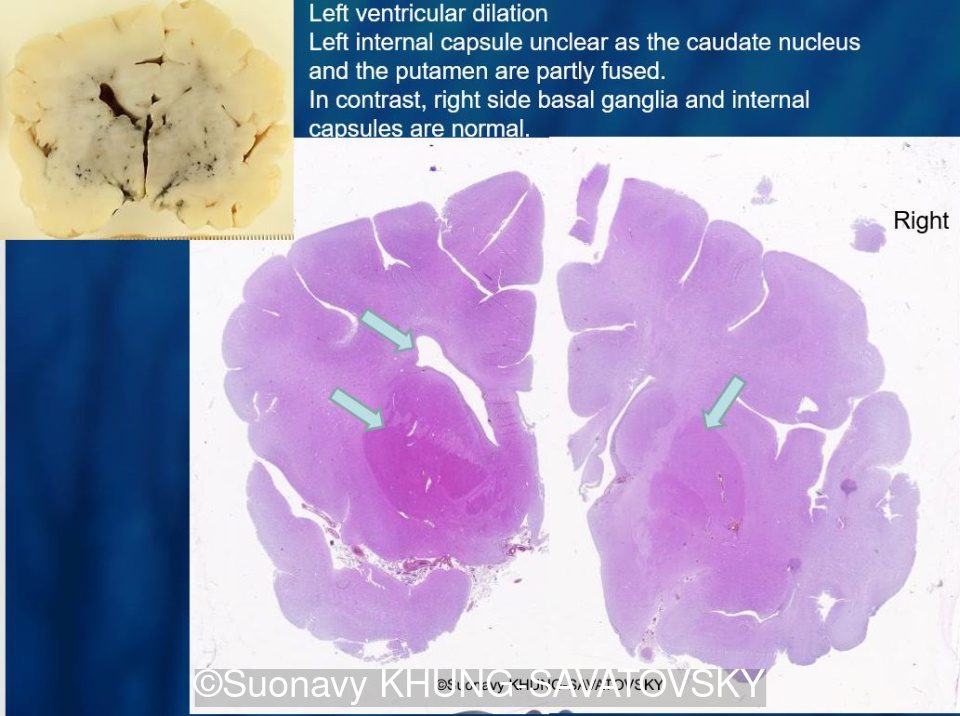
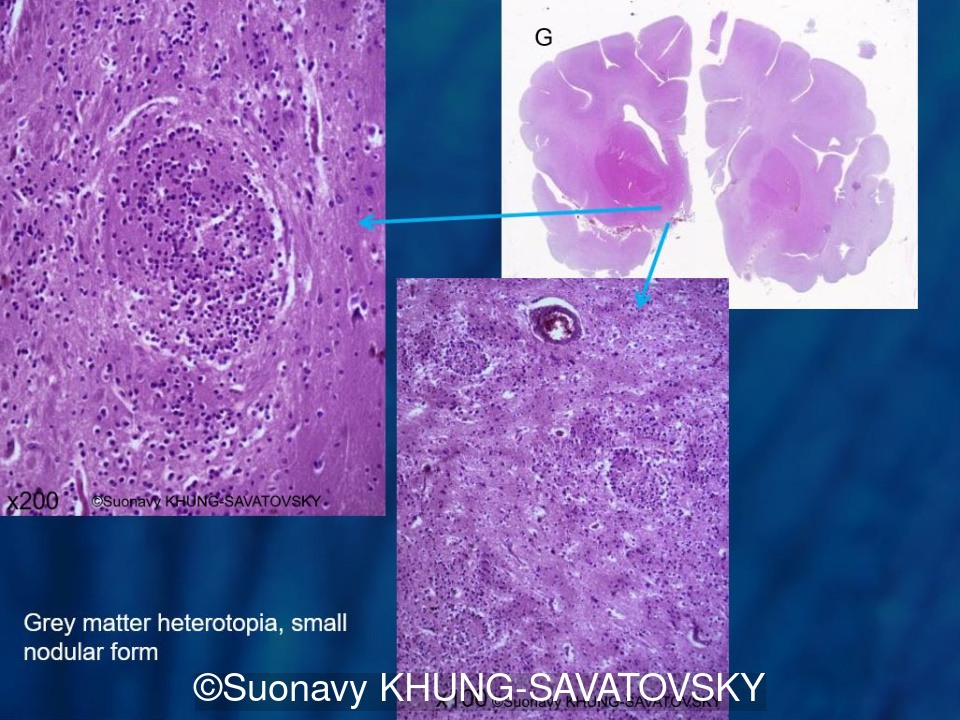
Fetal pathologic examination confirmed the MRI findings and found additional anomalies not initially identified by the MRI; both macroscopically and microscopically (Images 1-10 below). Macroscopic examination confirmed the asymmetric pons with left predominance and distortion of the anterior part of the interhemispheric fissure. Additionally, there was an unusual left cingulate sulcus unseen by the prenatal MRI. Microscopic examination of the pons found a paucity of neurons on the right side, and anarchic disorganized fiber tracts on the left side, hence the asymmetric appearance on imaging. It also found absence of red nucleus on the left side (?) compared to the right side, bilateral hypoplasia of the pyramidal tracts, and a few nodular supratentorial grey matter heterotopia not originally seen on the MRI. These findings were consistent with a Tubulinopathy.
After the pathological examination, a gene panel sequencing was performed, targeted at genes involved in tubulinopathies. In the TUBB3 gene, we found a heterozygotic variant of unknown significance ( c.156C>G (p.Asn52Lys) in the exon 2) but most likely pathogenic due to the abnormal brain findings. This variant was not found in the parents genome, and was therefore de novo.
Our final diagnosis was therefore a TUBB3-related Tubulinopathy (OMIM #614039)
Discussion
Tubulins are proteins composed of α and β-tubulin monomers, resulting in blocks of microtubules. Microtubules are part of the cytoskeleton and are involved in brain embryogenesis through their role in mitosis, intracellular transport, cell migration and neuron morphology. Mutations in the genes of tubulins TUBA1A, TUBB2A, TUBB2B, TUBB3, TUBB (TUBB5), or TUBG1 can affect microtubule stability, thus disrupt neuronal division and migration, leading to brain malformations. Most pathologic mutations are de novo [2]. The spectrum of tubulinopathies consists of non-syndromic cerebral malformations characterized by the association of cortical dysplasia and pontocerebellar hypoplasia, manifesting with global developmental delay, mild to severe intellectual disability, axial hypotonia, strabismus, nystagmus and occasionally, seizures and optic nerve hypoplasia [2].
- The severe prenatal form is characterized by voluminous germinal matrices, microlissencephaly, and a kinked brainstem
- A mild prenatal form is associated with non-specific features, including an asymmetric brainstem, distortion of the anterior part of the interhemispheric fissure with subsequent impacted medial borders of the frontal lobes, callosal dysgenesis, and a lack of Sylvian fissure operculation. In the absence of additional extra-cerebral anomalies, the combination of which is highly suggestive of tubulinopathies,
- Infectious foetopathies in the appropriate setting (for example microcephaly with pontocerebellar brainstem hypoplasia induced by Zika virus)
- 5p-deletion (cri-du-chat or cat's cry syndrome)
- True primitive pontocerebellar dysplasia described and classified by Barth PG (type 2 and type 4 linked to TSEN54 gene mutations are the most commonly identified prenatally)
- Kinked brainstem in Walker-Warburg syndrome (for example with type 2 lissencephaly also known as "overmigration")
- Brainstem dysplasia in KIAA1109 gene mutation which is often associated with arthrogryposis, heart and ocular defects
- The recently described entity "pontine tegmental cap dysplasia"
- Some congenital muscular dystrophies
- Some metabolic conditions such as Congenital disorders of glycosylation
References
[1] Cabet S, Karl K, Garel C, et al. Two different prenatal imaging cerebral patterns of tubulinopathy. Ultrasound Obstet Gynecol. 2021 Mar;57(3):493-497.
[2] Bahi-Buisson N, Maillard C. Tubulinopathies Overview. 2016 Mar 24 [Updated 2021 Sep 16]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2021. Available from: https://www.ncbi.nlm.nih.gov/books/NBK350554/
[3] Bahi-Buisson N, Poirier K, Fourniol F, et al. The wide spectrum of tubulinopathies: what are the key features for the diagnosis? Brain. 2014 Jun;137(Pt 6):1676-700. PMID: 24860126.
[4] Arrigoni, F., Romaniello, R., Peruzzo, D. et al. The spectrum of brainstem malformations associated to mutations of the tubulin genes family: MRI and DTI analysis. Eur Radiol. 2019 Feb;29(2):770-782.
[5] Kniffin CL. "Cortical dysplasia, complex, with other brain malformations 1; CDCBM1 #614039" OMIM. https://www.omim.org/entry/614039, Publish date 6/2011.
[6] Rasooly RS. "Tubulin, BETA-3; TUBB3 #602661." OMIM. https://omim.org/entry/602661, Publish date 5/1998.
I especially want to thank Dr Catherine Garel and Pr Laurent Guibaud for their speech on fetal brainstem pathologies at the fetal medicine congress in Montpellier in November 2019. Without their presentation, I honestly would not have thought about a tubulinopathy at this early prenatal stage. They particularly reported cases of tubulinopathies with prenatal MRI that had striking similar findings. I also thank them for their kind advice to our team on this peculiar case.
Thanks also to Dr Khung Savatovsky for keeping in touch with great pathology images. - Fabien Ho
Discussion Board
Winners

Lusine Karapetyan Russian Federation Physician

Umber Agarwal United Kingdom Physician

Ta Son Vo Viet Nam Physician